Abstract
Background: Inhibitors of Bruton's tyrosine kinase (BTKi) are approved and clinically used as indefinite treatments (until disease progression) irrespective of the depth of response in patients (pts) with CLL. Despite impressive, sustained efficacy, their ability (as single agent) to target residual disease clone post induction treatment in CLL is not well established. As indefinite treatment is associated not only with cumulative toxicity but also high cost and compromised quality of life, we investigated the benefit of time-limited maintenance treatment (3 years) with ibrutinib to assess the ability of a BTKi to further deepen antitumor response achieved post induction treatment.
Methods: CLL pts (n=35) who had residual disease (by clinical or molecular assessment) after primary induction treatment were enrolled on a prospective phase 2 trial (NCT02649387). MRD assessed in blood (PB)/bone marrow (BM), was determined by 8-color flowcytometry (1x10-4) and defined as ≥1 CLL cell per 10,000 leukocytes or ≥0.01%. Patient were eligible if they had detectable residual (non-progressive) disease within 1 year of completing induction and no prior ibrutinib (or other BTKi) treatment. Ibrutinib 420mg PO QD was given on days 1-28 of a 28-day cycle for up to 36 cycles. Clinical (iwCLL 2008 response criteria) and molecule response MRD (on PB) was done at baseline and then every 3 cycles while on treatment. BM-MRD evaluation was conducted only if PB-MRD was negative (on two separate timepoints 3 months apart). Monitoring of the PB-MRD was continued for 24 months after completing time-limited maintenance with ibrutinib. Cumulative toxicity was assessed using the CTCAE v4.0 and iwCLL criteria.
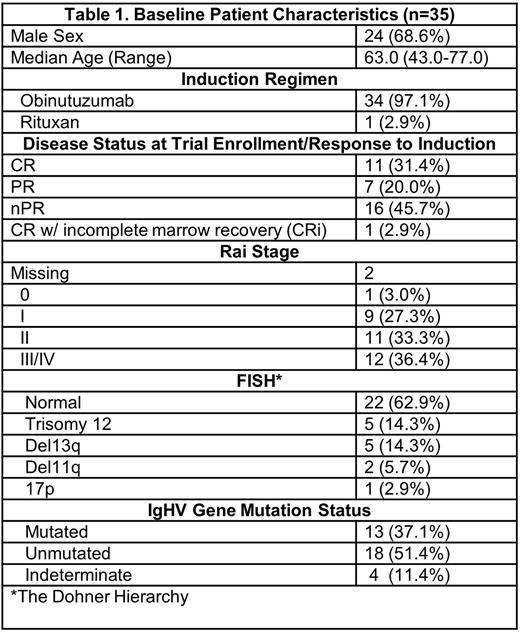
Results: Patient characteristics are summarized in Table 1. In 34 out of 35 (97%) patients, obinutuzumab alone was used as induction therapy. The median follow up is 38.6 months (range 0.9-73.2) and the median number of maintenance cycles completed is 34 (range 1-36). Of the 33 patients assessed for PB-MRD, 8 (24.2%) pts became MRD- on at one assessment. Five patients had BM-MRD assessment with 1 (2.8%) converting to MRD-. The median time to achievement of MRD- was 4.4 months (range 2.7-34.7). We noted that single agent ibrutinib was unable to deliver stringent MRD-negativity (defined as achievement of MRD- status both in PB and BM sustained over 2 consecutive evaluations at least 3 months apart).To assess if pre-maintenance response could impact MRD post-treatment; we note that out of the 8 pts that achieved MRD-, 2 (25%) started with CR and 6 (75%) with nPR/PR at registration. Deepening of response was noted in 5 pts (14.3%) after a median of 14 cycles [3 (8.6%) pts improved nPR to CR and 1(2.9%) went from nPR to CRi and 1 (2.9%) went from a CRi to a CR]. Nine patients proceeded to subsequent treatment with the median time to next treatment (mTTNT) of 32.5 m (range: 12.1 - 61.0). One patient died due to a failure to thrive and acute kidney injury on dialysis and six pts have progressed. The median PFS for the entire cohort was 41.1 m (95% CI: 39.8 - NE). The most commonly reported adverse events (AEs) (seen in >10% pts) regardless of attributions were thrombocytopenia (24 pts; 68.6%), anemia (12 pts; 34.3%), neutropenia (9 pts; 25.7%), lymphopenia (9 pts; 25.7%), hypertension (9 pts; 25.7%), leucopenia (6 pts; 17.1%), fatigue (5 pts; 14.3%), headache (4 pts; 11.4%), and skin infections (4 pts; 11.4%). Atrial fibrillation (AFib) was noted in 3 pts (8.6%).
Conclusion: We conclude that BTK targeting with ibrutinib alone although able to clear PB-MRD (24.2%), had limited efficacy to eradicate BM-MRD (2.8%) demonstrating a differential impact in these two disease compartments. While the rate of complete MRD eradication was not necessarily high (when compared to recent data with BTKi/BCL2i combination regimens) MRD-kinetics over time demonstrated progressive decrease in disease burden and response improvement with maintenance treatment. The sequential single agent treatment approach was well tolerated, and the time-limited therapy aimed at minimizing cumulative toxicity seemed to be effective in lowering rate of Afib (compared to historical control ~16%). This data warrants further investigation of time-limited maintenance therapy with newer, more favorably tolerated BTKi and/or Bcl2i post induction treatment(s) to optimize response vs. cumulative toxicity. Further evaluation of sequential time-limited treatments in CLL is warranted.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal